Japanese researchers have proposed a novel method to produce blue hydrogen directly inside oil reservoirs by using residual oil that is usually left behind after extraction and considered too costly to recover. Instead of bringing this oil to the surface, the process takes place deep underground, where heat, oxygen, water, and specially injected mineral particles work together to convert the remaining oil into hydrogen-rich gas within the rock itself. By relying on existing oil recovery techniques and natural underground conditions such as pressure and temperature, the approach reduces the need for complex surface facilities and reimagines old oil fields as potential sources of cleaner energy.
Turning unused oil into hydrogen underground
After oil fields are produced for many years, a large amount of oil still remains trapped in the rocks. This leftover oil is called residual oil. In most cases, it is not recovered because doing so costs more money than the oil is worth. The proposed method focuses on using this residual oil as a feedstock for hydrogen production.
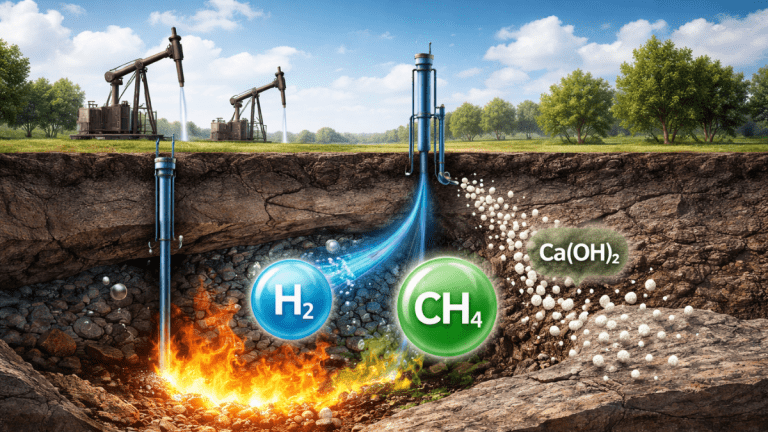
The process begins after enhanced oil recovery operations are completed. At this stage, tiny mineral particles are injected into the reservoir. These particles are made of nickel oxide and calcium hydroxide. They are extremely small, allowing them to spread through the tiny spaces in the rock.
Next, oxygen is injected into the reservoir. This oxygen ignites a portion of the remaining oil. This controlled burning process is known as in-situ combustion. The goal is not to burn all the oil but to generate very high temperatures underground.
As temperatures rise, the remaining oil starts to break down. The oil turns into lighter gases such as methane. These gases then react with water vapor present in the reservoir. This reaction produces hydrogen, which becomes part of a hydrogen-rich gas mixture. All of this happens underground, without lifting the oil to the surface. The rock formation acts like a natural reactor, handling extreme heat and pressure that would otherwise require expensive industrial equipment.
How minerals boost hydrogen and trap carbon
Nickel oxide plays an important role during heating. When temperatures rise, it releases oxygen. This extra oxygen strengthens the combustion process and pushes temperatures higher. As the nickel oxide releases oxygen, it turns into metallic nickel.
Metallic nickel is useful because it acts as a catalyst. A catalyst is a substance that speeds up chemical reactions without being used up. In this case, nickel helps hydrocarbons and methane react with steam more efficiently. This reaction produces more hydrogen.
However, there is a downside. Faster reactions also lead to more carbon dioxide being formed. Carbon dioxide is a greenhouse gas, and reducing it is a key goal in blue hydrogen production. This is where calcium hydroxide becomes important. Calcium hydroxide reacts differently from nickel oxide. It also helps with steam reactions, but it has an extra benefit. It captures carbon dioxide as it forms.
When carbon dioxide meets calcium hydroxide, a chemical reaction occurs. The carbon dioxide becomes solid calcium carbonate. This solid material stays trapped inside the rock pores and does not rise to the surface as a gas.
This means carbon is locked underground instead of entering the atmosphere. At the same time, hydrogen production increases. In laboratory tests, adding calcium hydroxide raised the hydrogen share in the gas mixture to 42 percent. Without it, hydrogen levels were about 9 percent lower. By producing hydrogen and trapping carbon in solid form, the process meets the definition of blue hydrogen. Blue hydrogen is hydrogen made from fossil fuels while capturing or storing carbon emissions.
High energy conversion inside oil reservoirs
To test the idea, scientists recreated underground conditions in a laboratory. They used a reactor filled with sand, oil, water, and minerals. The mixture was heated to temperatures between 400 and 800 degrees Celsius, similar to conditions expected during in situ combustion underground.
At higher temperatures, especially near 800 degrees Celsius, the results were strong. Up to 70 percent of the energy stored in the original oil was converted into useful fuels. These fuels were mainly hydrogen and methane. This level of energy conversion is significant. It shows that leftover oil, which normally has little economic value, can still deliver large amounts of usable energy.
US increases enforcement of Venezuelan oil restrictions through military support
The gas produced during the process contains both hydrogen and methane. Methane can be used directly as fuel or further processed. Hydrogen can be separated and used in power generation, industry, or transport.
Because calcium hydroxide traps much of the carbon dioxide underground, the carbon footprint of the produced gas is lower than traditional fossil fuel processes. The trapped carbon stays in solid form within the reservoir rocks, reducing the need for surface carbon capture systems.
The process relies heavily on existing oil recovery knowledge. In-situ combustion and underground fluid injection are already familiar to the oil and gas industry. This makes the concept easier to understand and potentially easier to adapt within existing fields.
By combining heat, minerals, water, and residual oil underground, the method shows how old energy resources can be transformed into cleaner fuels without extensive surface processing.